A critical part of any oilfield production processing is oil and water separation. Although some highly effective demulsifier products for this purpose have been developed for a number of production scenarios, the application of demulsifiers to “heavy” oils remains problematic. However, the work described below has led to the development of a tailored range of demulsifiers to add to the relatively small number of effective chemistries applicable across the growing demands of heavy oil production activity.
Theoretical models show that the critical parameters of how chemical demulsifiers break crude oil emulsions are associated with the rheology of the oil/water interface. The work undertaken was to determine whether such correlations are justified in real systems by measuring the rheological parameters associated with the oil/water interface and correlating them with demulsifier performance. The principle of this model was then used to design more efficient demulsifier molecules. All demulsifiers were evaluated individually for comparison and not as a demulsifier blended package.
To meet the needs of field applications, the desired demulsifiers must be very interfacially active and be able to displace the surfactants in crude oil at a use concentration as low as 10 ppm. A range of novel heavy oil demulsifiers has been developed with a wide demulsification chemistry portfolio, including resins, polymerics, and esters to optimize and further develop more efficient molecules.
Experimental Work
Interfacial Tension (IFT). Demulsifiers that showed good performance in the IFT testing were further evaluated in a range of crude oils with a range of API gravities, water cuts, compositions, and regions of origin.
Relative Solubility Number (RSN). The relative solubility number of a demulsifier is a measure of its solubility properties. This is a key factor in demulsifier selection, because solubility properties dictate whether the chemical will perform effectively as a surface-active agent at the oil/water interface. The following physical properties, pour point, viscosity, density, and pH, were measured to provide an indication of how the products could be handled in the field.
Turbiscan Analysis. A Turbiscan laboratory instrument, which replicates on-field bottle testing, was used to evaluate the demulsifiers. This method enables the acceleration and documentation of an aging test for an in-depth understanding of the destabilization mechanisms (creaming, sedimentation, flocculation, and coalescence) of emulsions.
Results and Discussion
A list of the initial chemistries and their physical properties is shown in Table 1.
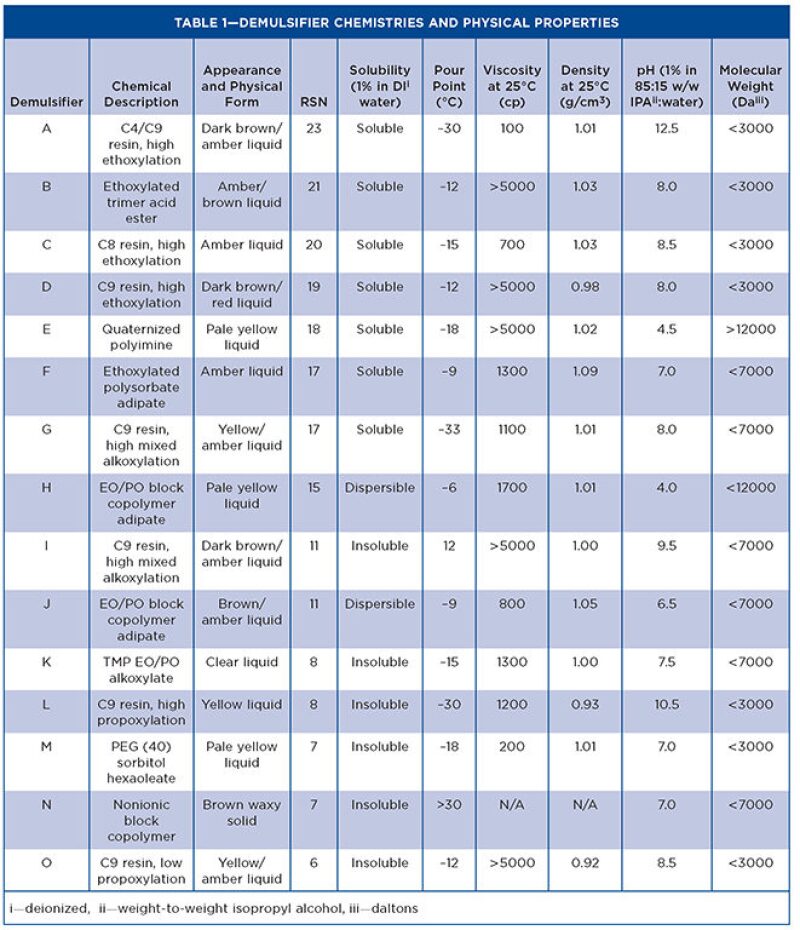
To establish information regarding the equilibrium adsorption of the demulsifier materials at the oil/water interface, the IFT (mN/m) was measured over time (sec) for the demulsifiers. Figs. 1 and 2 show the IFT analysis for the demulsifiers listed in Table 1. The graphs show that the steeper the gradient, the faster acting the demulsifier, and the lower the equilibrium IFT, the more effective the demulsifier.
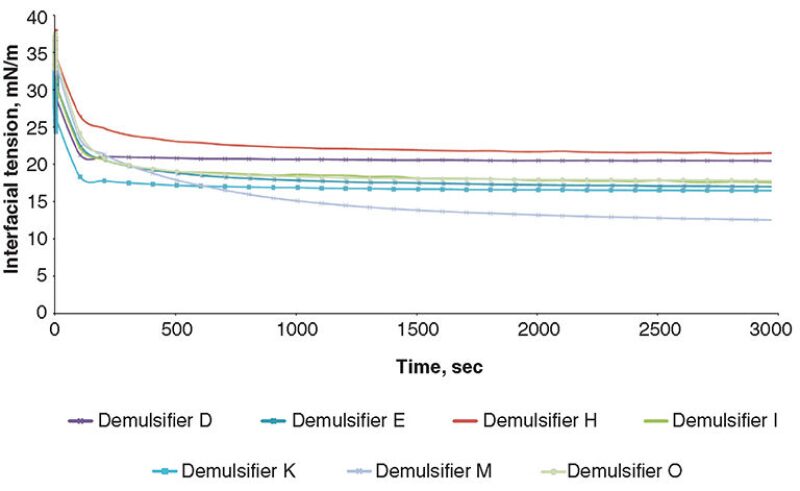
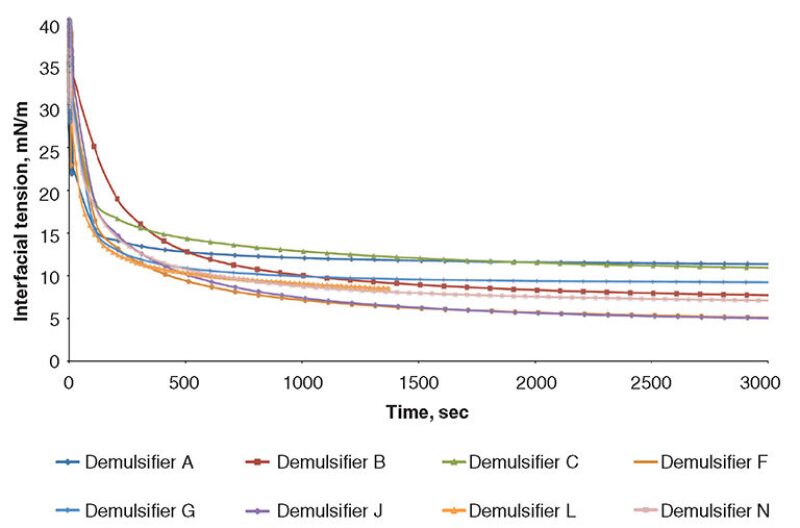
The results show that an increased surfactant adsorption time results in a reduction in the value of the IFT. This also leads to an increase in the value of the interfacial elastic modulus. The demulsifiers were then further evaluated in the Turbiscan instrument, in some crude oils with a range of API gravities and water cuts. Three heavy crude oils with different gravities and basic sediment and water (BS&W) levels were used for screening the demulsifiers and the details. The crude oils are given in Table 2.
Tables 3 through 5 show the Turbiscan results from the evaluation with the crude oils.
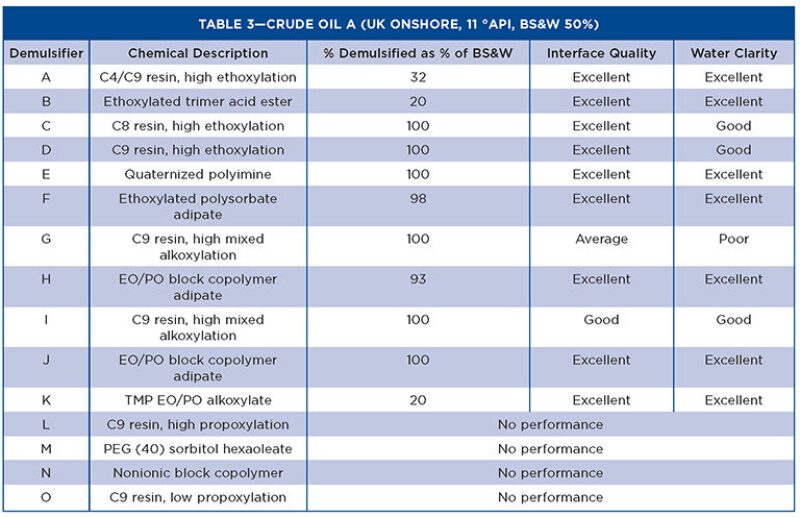
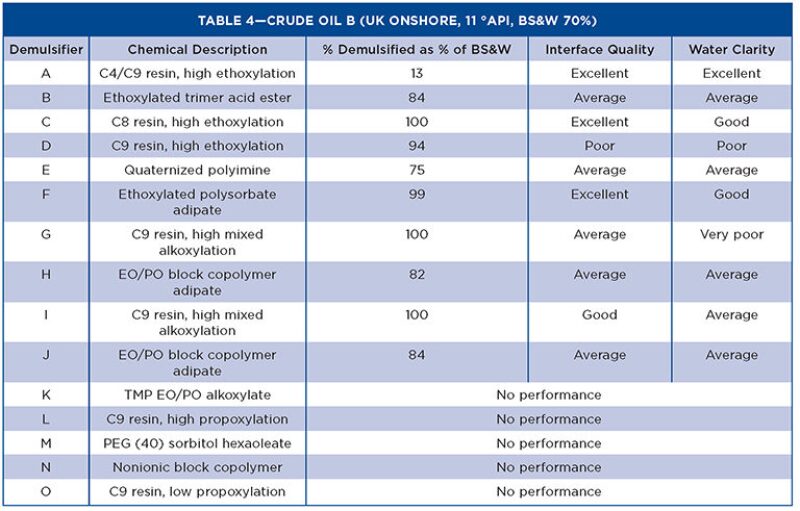
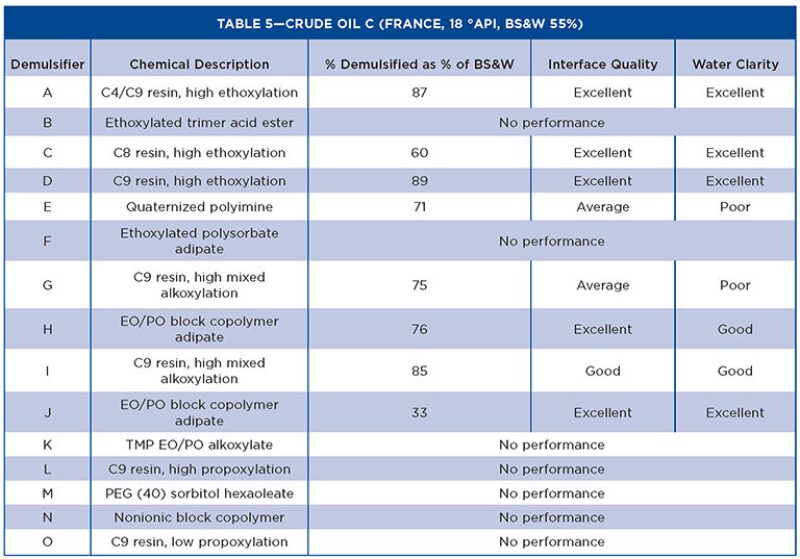
The characteristics affecting demulsifier performance show that molecular weight, RSN, and functional groups are the keys to providing good separation of the water and oil in heavy oils. The IFT test gives a bias toward high RSN demulsifiers, which are stronger water droppers. This is mirrored by the Turbiscan tests in the crude oils, as all demulsifiers <8 RSN did not exhibit any separation. However, when the RSN was increased slightly (~11), the performance improved significantly regardless of the chemical backbone of the molecule. This is related to the extent of alkoxylation, and the products that have high mixed alkoxylation levels have better demulsification properties than single alkoxylated products.
In general, the more water soluble demulsifiers show rapid interfacial adsorption and high values of interfacial elastic modulus. The value of interfacial elastic modulus is related to the strength of interaction between molecules at the oil/water interface. Bulky hydrophobes based on resins, which cannot pack well at the interface, have lower elastic modulus and promote coalescence. With higher molecular weight chemistries, the interfacial adsorption rate decreases, the interfacial activity increases, and the interfacial elastic modulus decreases. Therefore, for higher molecular weight demulsifiers, the initial effect is slower but more effective overall.
The other physical properties evaluated in the study show no correlation with performance, but are required for the user to understand how to handle and use the demulsifiers in field conditions. The chemistries chosen were varied to assess surfactants other than those based on nonyl phenol resins. Although the nonyl phenol-based demulsifiers are still the best performers, other chemistries have shown suitable demulsification performance. In particular, an ethoxylated polysorbate adipate (patent pending) performs well in heavy oil, while classified as yellow and inherently biodegradable for use in the North Sea.
Internal development work was also done to evaluate resins with longer alkyl chains as resin alkoxylate demulsifiers. From this work, it was found that the ethylene oxide:propylene oxide mix and resin:alkoxylate ratio are more important for demulsification performance than the type of resin used.
Customer trials with demulsifiers were executed and the leading products correlated with the performance in the laboratory. However, a formulation of several demulsifiers is commonly used to obtain optimum field performance.
Conclusions
Leading-edge techniques have been used that measure demulsifier interfacial properties, measure how these properties relate to demulsifier performance, and evaluate how a range of demulsifiers perform in heavy crude oils. The elements of molecular structure that determine adsorption properties have been discussed, and this understanding has guided the development of new demulsifier types with improved performance. Although the nonyl phenol resin alkoxylates are the best performing products, a variety of chemistries can be used in heavy oil, including ethoxylated polysorbate adipates.
In field situations, the most effective demulsifiers are likely to be a formulation of high and low molecular weight materials. The high molecular weight species occupies a large area at the interface, which reduces the surfactant concentration, whereas the lower molecular weight materials will aid rapid relaxation of the IFT. The difficulty in designing better performing demulsifiers lies in balancing these various parameters, some of which are in opposition.
Acknowledgments
The authors would like to thank Croda employees involved in helping with the article. The authors also wish to thank Recherche Exploitation Produits and Star Energy for supplying materials, and Teclis and Fullbrook Systems for their technical support.

